Research Programme: Biophysics and Biomedical Engineering
Principal Investigator: Prof. Nicola Rosato

Among the scientific interests of Prof. N. Rosato is cancer nanomedicine, a research field recently stemmed from the combination of nanotechnology with cancer medicine. Cancer nanomedicine aims at improving the efficacy of anti-cancer therapeutic payloads by controlling their delivery with spatiotemporal accuracy by using nanoparticles (NPs) [1]. Prof. Rosato has been working on the development of NPs aimed at the treatment of malignancies for more than 10 years. In one study developed in collaboration with the Sanford Burnham Prebys Medical Research Institute (La Jolla, USA), Prof. Rosato’s group assessed the ability of carbon-based NPs to deliver gene inhibitors into CD4+CD25high FoxP3+ regulatory T cells residing in the tumor microenvironment and silence a model gene in vivo [2]. In 2017, one of the members of his research group (Prof. M. Bottini) was awarded with a fellowship from the Chinese Academy of Sciences President’s International Fellowship Initiative (CAS-PIFI) to visit the Laboratory of Controllable Nanopharmaceuticals led by Prof. X.J. Liang at the CAS. During his visit, Prof. Bottini was involved in projects aimed at the development of novel types of NPs for the controlled release of therapeutic cargo in tumors and inflamed joints. In the field of cancer, an innovative theragnostic albumin-based NP enabled the eradication of tumors in mice via a combination of photothermal-, photodynamic- and chemo-therapy [3,4]. Additionally, a degradable core-satellite NP was developed to enable monitoring by fluorescence and photoacoustic dual-modal imaging in a real-time noninvasive manner the enzyme-activatable release of doxorubin (DOX) in the tumor microenvironment [5]. These studies clearly show the high level of expertise reached by Prof. Rosato’s group in the field of cancer nanomedicine in the context of active collaborations with several international research institutions.
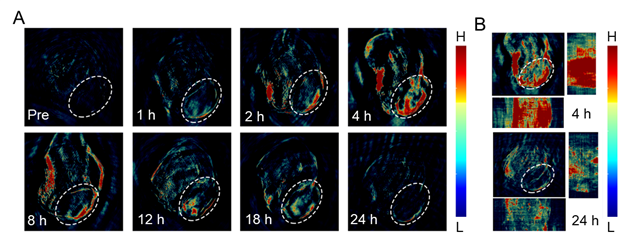
Development of an enzyme-activated anti-cancer approach
The approach is based on core-satellite architecture gelatin nanoparticles (gelatin NPs or GNPs) loaded with “core” near-infrared (NIR)-emitting indocyanine green (IGNPs) and coated with “satellite” CuS NPs (CuS/IGNPs). The combination of core-satellite architecture and degradability in response to proteases overexpressed in cancer tissues enable the in real-time monitoring of NP trafficking and drug release by means of a combination of photoacoustic (PA) and fluorescence (FL) signals. Intact NPs displayed an undetectable FL signal arising from “core” ICG, in virtue of the shielding effect of CuS NPs, and a strong PA signal arising from CuS NPs. Upon accumulation of the NPs in cancer tissues, overexpressed proteases trigger the degradation of the gelatin matrix and the release of ICG, which translates in an increase in the FL signal. The kinetics of enzyme-activated drug release was assessed for NPs both dispersed in a saline buffer and administered to tumor-bearing mice locally and systemically. Computer simulation was also used to derive a model of drug release from degradable NPs. [5]