Research Programme: Characterization of leukemic stem cells in FLT3-ITD-positive acute myeloid leukemia
Principal Investigator: Dr Serena Travaglini

Scientific Interest
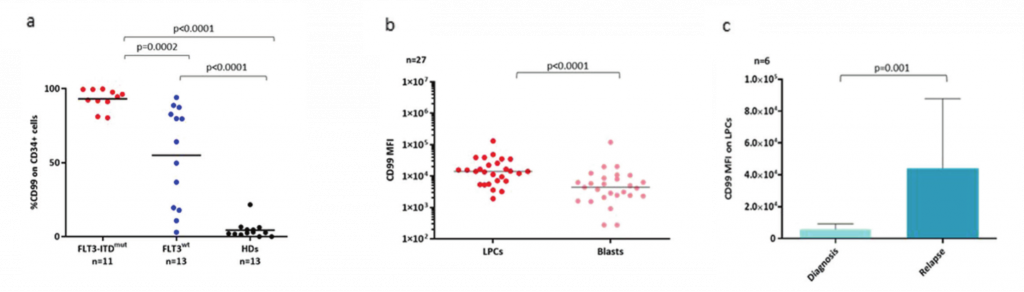
It has been consistently shown the negative prognostic impact of FLT3-ITD mutations, associated with reduced overall survival and a high rate of disease recurrence, partly due to the persistence of FLT3-ITD-expressing LSCs after initial AML treatment. The CD34/CD123/CD25/CD99+ leukemic precursor cells (LPCs) phenotype is identified as a strong predictor of FLT3-ITD mutation and disease recurrence. However, the lack of thorough characterization of these cells hinders the selective eradication of LSCs, a major challenge in AML treatment. Despite the availability of targeted therapies, relapse rates remain high, emphasizing the urgent unmet need for novel therapeutic interventions. In a recent study, Travaglini et al. identified CD99 as a reliable molecular marker for FLT3-ITD+ LSCs and a potential predictor of disease progression, suggesting its potential as a promising therapeutic target. Her work delves into a deep characterization of FLT3-ITD+ LSCs, aiming to identify novel therapeutic targets and design patient-tailored targeted therapies that can induce complete remission and improve patient outcomes.
Current Research
Her current research focuses on the comprehensive characterization of the unique CD34/CD123/CD25/CD99 immunophenotype of FLT3-ITD mutated LSCs, allowing for a detailed examination of the minute cell fraction responsible for recurrence and resistance to conventional treatments. Through an in-depth analysis of the transcriptome profile of FLT3-ITD mutated LSCs, they unveiled the activation of signaling pathways which could cooperate with oncogenic FLT3-ITD, promoting leukemia development and resistance. Therefore, understanding these mechanisms is crucial for designing LSC-directed therapeutic strategies to overcome treatment resistance.
Furthermore, in vivo utilizing murine models have unequivocally established the stem-cell-like properties of the identified population, highlighting the critical importance of comprehensive characterization in designing effective molecularly targeted therapeutic strategies. These findings provide valuable insights into the molecular intricacies of FLT3-ITD mutated LSCs, while also opening up avenues for innovative therapeutic interventions and a deeper understanding of disease relapse and treatment resistance.
Relevance to cancer biology
The current study aims to comprehensively characterize the FLT3-ITD stem cell compartment, both in vitro and in vivo, to provide the rationale for effective combination strategies, and to incorporate novel biomarkers associated with dynamic treatment resistance.